 When I was a wee lad back in Scotland one of my favourite films was a movie called `Fantastic Voyage’.
When I was a wee lad back in Scotland one of my favourite films was a movie called `Fantastic Voyage’.
Based on an Issac Asimov novel it’s about a group of scientists who, along with their hi-tech sub are miniaturised and injected into the body of an emminent scientist. Their mission:- to perform some very targeted brain surgery from within using lasers.
(The film is often most remembered by film reviewers for a scene where our hero has to rip ‘giant’ (to them) `phagocytosing’ white blood cells from a wetsuit-clad Raquel Welsh. At the time I was way too young to understand why `that scene’ was so appealing to grown-ups! Especially when there were so many other cool scenes of them travelling through the blood stream, lungs, inner ear and finally in the brain surrounded by hanging neurones!).
Anyway, when I read this article on `Optogenetics’ -a new technology that potentially allows scientists to switch individual neurones on and off by means of light – the movie leapt into my mind and I became intrigued to read on.
It’s a facinatating concept and another example of 21st century ingenuity from the rapidly expanding world of nanotechnology.
Check it out here:-
http://the-scientist.com/2011/07/01/optogenetics-a-light-switch-for-neurons/
or read full article here
http://the-scientist.com/2011/07/01/the-birth-of-optogenetics/
P.S. For all the film buffs out there, a remake of ‘Fantastic Voyage’ in rumoured to be one of James Cameron’s latest projects.

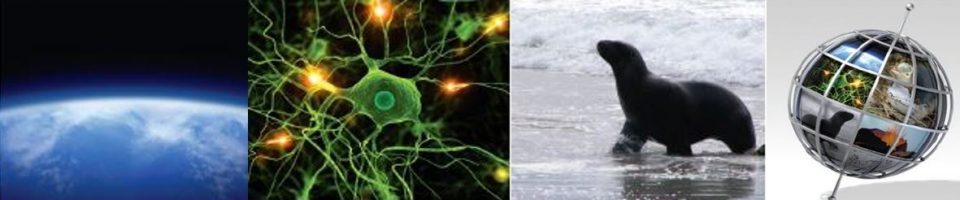
 There’s no more stimulating way to end a busy week than a good scientific controversy!
There’s no more stimulating way to end a busy week than a good scientific controversy! A more sedate way to get to grips with Protein synthesis
A more sedate way to get to grips with Protein synthesis
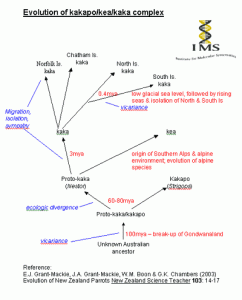 Struggling to get your head around role of polyploidy in speciation, adaptive radiation and such like?
Struggling to get your head around role of polyploidy in speciation, adaptive radiation and such like? Link to a Chemistry Home lab site with 16 experiments ranging from sampling air quaility to energy efficiency, to water and soil testing, to making snow, and `ghost-buster’ slime. Step by step instructions with good illustrations.
Link to a Chemistry Home lab site with 16 experiments ranging from sampling air quaility to energy efficiency, to water and soil testing, to making snow, and `ghost-buster’ slime. Step by step instructions with good illustrations.




 This achievement standard
This achievement standard 


Recent Comments