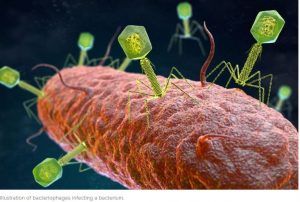
 With all that is going on around us, all across this beautiful planet of ours and the word `virus’ dominating everything we see, hear, read in the media at the moment I found myself thinking “Are all viruses bad? Are there any useful, beneficial viruses? And why ARE bats such good carriers off so many nasty diseases – over 60 of them!”
With all that is going on around us, all across this beautiful planet of ours and the word `virus’ dominating everything we see, hear, read in the media at the moment I found myself thinking “Are all viruses bad? Are there any useful, beneficial viruses? And why ARE bats such good carriers off so many nasty diseases – over 60 of them!”
It seems highly likely that viruses do play a substantial part in maintaining a healthy body. You have probably heard of our `microbiome’ but what about our ‘virome’
Read more here:
How ‘good’ viruses may influence health
Here:
Not All Viruses Are Bad For You. Here Are Some That Can Have a Protective Effect
And here:
Viruses Don’t Deserve Their Bad Rap: They’re The Unsung Heroes You Never See
[In the embedded Ted-X talk in this link, the always engaging, Peter Pollard also illustrates some illuminating facts about how much C02 freshwater ecosystems pump into the atmosphere, due in part to viruses… everything is linked! ]
And on the question of why bats are such good carriers of disease.
Check out:
Why Do Bats Transmit So Many Diseases?
BEFORE YOU CLICK- Spend a minute thinking about what you know of bat behaviour and basic physiology …. (The answer may take flight in your head…(hint))
However, when all is said and done our thoughts are all on one virus at the moment.
Ever wondered how the tests for Coronavirus work?
May you never have to take one!
P.S.
Need a refresher on just exactly what viruses are and how they work?
Check out this great Khan Academy Tutorial on Viruses and the text Q&A that follows.
Stay safe
Be patient












 There’s no more stimulating way to end a busy week than a good scientific controversy!
There’s no more stimulating way to end a busy week than a good scientific controversy! Link to a Chemistry Home lab site with 16 experiments ranging from sampling air quaility to energy efficiency, to water and soil testing, to making snow, and `ghost-buster’ slime. Step by step instructions with good illustrations.
Link to a Chemistry Home lab site with 16 experiments ranging from sampling air quaility to energy efficiency, to water and soil testing, to making snow, and `ghost-buster’ slime. Step by step instructions with good illustrations.





Recent Comments