Research Interests
(a) Unsymmetrical polynucleating macrocycles
Many metalloproteins have polynuclear active sites and many contain more than one type of metal binding environment (eg. ascorbate oxidase, a tricopper metalloprotein). Our successful design and synthesis of unsymmetrical multinuclear macrocycles is therefore a very promising development. These macrocycles should give us access to good metalloprotein model complexes and to complexes which are of interest in their own right. We are preparing a series of tricopper complexes as structural models for ascorbate oxidase.
(b) Heterocycle-containing ligands – towards nanoswitches and memory devices
The use of pyridazine as a two atom bridge in transition metal complexes is of much interest. This is due to both its ability to mediate significant magnetic interactions between metal centres, and to the fact that it generally results in the observation of positive reduction potentials for the Cu(II) to Cu(I) process, in contrast to the analogous phenol-bridged complexes.
We are using 3,6-diformylpyridazine, and related heterocycles, as starting materials for both acyclic and macrocyclic ligands. A variety of multinuclear complexes are being prepared and structurally characterised. Interesting electrochemistry is a feature of the dimetallic, doubly-pyridazine bridged, transition metal complexes. The exciting magnetic behaviour is being investigated in collaboration with Prof. K.S. Murray (Monash, Australia) and may lead to the development of useful nanocomponents (switches/memory devices)

X-ray crystal structure and magnetism of [CoII2L(NCS)2(SCN)2], Brooker, S., Plieger, P.G., Moubaraki, B. and Murray, K.S., Angewandte Chemie International Edition, 38, no. 3, front cover feature (1999), 408-410.
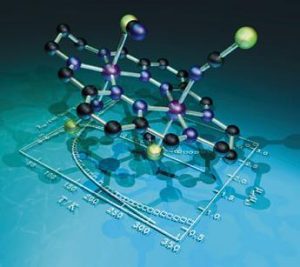
(c) Self-assembly of communicating arrays of transition metal ions
The structure of the first macrocyclic grid complex is shown below. This Schiff base macrocycle resulted from the 2+2 condensation of 3,6-diformylpyridazine and 1,3-diaminipropane.
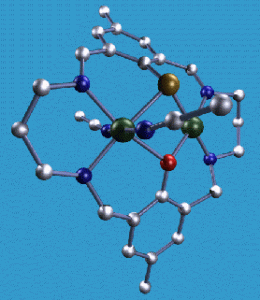
(d) Thiolate-containing ligands – models for hydrogenases (H2 = 2H+ + 2e–)
Thiolate donors are important in the active sites of many metalloproteins. The chemistry of first row transition metal thiophenolate complexes has not been extensively studied due to problems of polymerisation and/or disulfide formation. Our results with 2,6-diformylthiophenol are very exciting, with several nickel thiolate complexes sent to Prof. S.P. Cramer (Berkley, USA) for XANES and EXAFS studies as they are good model complexes for the [Ni,Fe] hydrogenase metalloproteins. In addition, reversible redox processes have been observed and are being further investigated. For example, EPR studies are being done in collaboration with Prof. A. McAuley (Victoria, Canada). One macrocyclic ligand in particular is very unusual in that it leads to adjacent square planar and octahedral nickel(II) ions despite providing potentially identical binding sites.

X-ray crystal structure of Ni2L2(MeCN)22+, S. Brooker and P.D. Croucher, J. Chem. Soc., Chem. Commun., 1995, 2075-2076.
(e) The oxygen evolving complex of green plants (in collaboration with Dr J. Eaton-Rye, Biochemistry)
Deprotonated amides are strong sigma donors and when coordinated to transition metals can stabilise high oxidation states. In this project amides are being incorporated into polynucleating ligands in order to prepare complexes with interesting redox properties. The tetramanganese metalloprotein responsible for 2H2O ® O2 in Photosystem II (Oxygen Evolving Complex) is one of the metalloproteins targeted in this project.
(f) lanthanide cages as a new generation of luminescent ‘probes’, in collaboration with Drs Jean Fleming (Anatomy and Structural Biology) and Sally McCormick (Biochemistry).
The interface between chemistry and biology is currently one of the most active and challenging areas of science. The chemical synthesis of non-radioactive reagents for use as labels for large biological molecules is of intense interest, as many of the radioactive labels used currently are expensive, hazardous, short lived, and have limited sensitivity.
Lanthanide complexes have the advantage of being non-radioactive and therefore provide a very attractive alternative. Those with long-lived luminescence lifetimes are particularly suited to development as stable macromolecular probes in biological applications such as research, diagnostics, biotechnology and the pharmaceutical industries.
Lanthanide luminesence is prevented by the binding of water molecules so the challenge is to develop ligands which totally encapsulate the lanthanide ions such that they ‘see’ no water even when dissolved in water. Our new ligands should wrap up the lanthanide ions better than ever before and provide a means of attaching the labels to DNA or proteins.