It is evident that there is asymmetry in the brain between males and females. Where the previous pages have explored the general sex differences of the brain and the hypothalamus, particularly using mammalian animal models, this article will explore, and provide examples of, the sex differences of the brain of non-mammalian vertebrates. This research is important in elucidating the biological basis of learned vocalization behavior as well as the sexual dimorphisms of such behaviors.
Zebra Finches (Taeniopygia guttata)
Zebra finches (Figure 1.) are songbirds. Named primarily due to their characteristic behavior, singing, songbirds are actually a taxonomic group. In particular, songbirds are thought to correspond to the specific order, Passeriformes, and suborder, Oscine [1]. Oscine passerines have a specialized neural circuit called the song-control-system. This song-control-system arises from the enhancement of existing vocal control circuits that can be observed in non-oscine avian species [2].
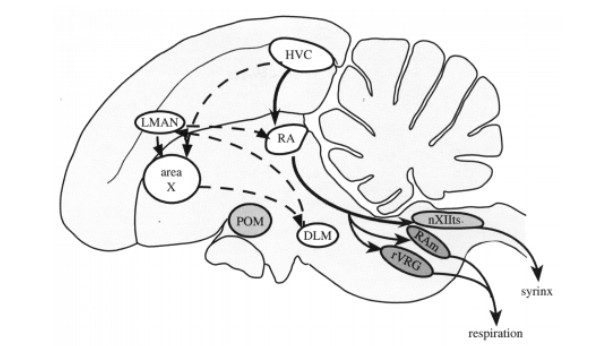
Figure 2: Schematic of the song-control system of songbirds. The caudal motor pathway (black arrows) directly connects HVC to RA and then to the motor neurons innervating the syrinx. The anterior forebrain pathway (dotted arrows) shows the indirect connection of the HVC to the RA. First signaling through area X then to the dorsolateral thalamic nucleus (DLM) then to the lateral magnocellular nucleus (LMAN).
Early studies examining Nissl-stained sections of brains of songbirds (zebra finches) noted that there were distinct volume differences in the key song nuclei of the song-control-system between male and female sections. That is, male zebra finch song nuclei, the HVC (proper name), robust nucleus of the arcopallium (RA) (Figure 3.) and area X of the medium striatum, were markedly larger than that of female zebra finch song nuclei [3,4]. These differences in the brain morphology are evident in the behavior of zebra finches as only the males sing to court female mates. To rephrase, male zebra finches sing to court mates whereas female zebra finches do not sing at all, and so this sex difference in behavior is correlated to the difference in volume of key song nuclei between males and females.
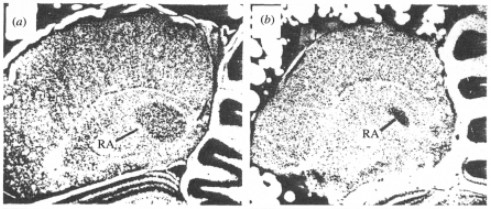
Figure 3: Nissl-stained sections of zebra finch RA. (a) Male zebra finch RA. (b) Female zebra finch RA. Male RA significantly larger than female RA.
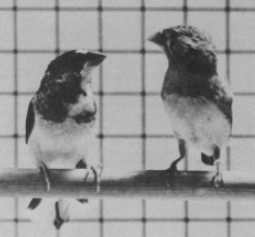
Figure 4: Female zebra finch (right) was treated with estradiol as a chick and treated with testosterone as an adult. Shown courting a Bengalese finch (left).
The classical model of sex difference in the brain is described by the effect that the differing hormonal control of each sex will result in differing morphologies in the brain. This concept was demonstrated in a study where a female zebra finch was treated with estradiol as a chick and treated with testosterone as an adult. These hormonal treatments activated song and courtship behavior in the female zebra finch (Figure 4.) [5]. Furthermore, three sections of the HVC were examined in three zebra finches: male, female, and female treated with estradiol. The difference in volume of the HVC between the male and untreated female sections were stark, as previously discussed. However, the hormonally treated female HVC closely resembled the male section in volume. Thus, suggesting that the sex difference in brain morphology and behavior is hormonally modulated [5].
Bay Wren (Cantorchilus nigricapillus):
While both being songbirds, bay wren and zebra finches have distinct differences. Taxonomically, bay wrens are non-oscine passerines unlike zebra finches. Moreover, there are no distinct sexual differences in plumage and body in bay wrens, unlike the distinctly different plumage pattern in zebra finches. However, the difference in singing behavior is likely of most interest. That is, male and female bay wrens engage in elaborate vocal duets. As such, both male and female bay wrens possess repertoires of song types used in duets [6].
Histological sections comparing the volumes of key song nuclei between male and female bay wren showed that there were no statistically significant differences between the volumes of the key song nuclei between both sexes [6]. This is consistent with the previous findings from the zebra finches. That is, due to greater song repertoire in females, that is comparable to males, it is logical that there would be little inter-sex difference in the key song nuclei of bay wrens. Using a broader view, bay wrens seem to be an inverse of the brain volume distribution of zebra finches. As zebra finches females do not sing and therefore have greatly diminished song-control-system nuclei; bay wrens females sing almost as much males and therefore have nuclei of similar volume.
Bay Wren Duet:
Crows (Corvus corone & C. macrorhynchos):
Avians have a different mode of sex determination than mammals. There are two primary difference between mammalian and avian sex determination. That is, avian have homogametic males (ZZ) and heterogametic females (ZW), also avians lack an SRY gene [7]. A study examining European crow (C. corone) brain gene expression found that there is a lack of dosage compensation of the Z chromosome [8]. That is, there is little regulation on the product of the second Z chromosome leading to more Z-linked genes being expressed in males than in female avians. There is further nuance of sex determination which outside the scope of this article but is explored here. Regardless, the novel model of the European crow was used to support the idea that avian sex determination is primarily dictated by sex-biased expression of Z-chromosome genes. The evidence of which originated from examining brain material [8].
Large-billed crows (Corvus macrorhynchos) show significant sexual difference in key song nuclei such as, HVC, RA, and area X [9]. This is morphologically similar to zebra finches [3], although male and female crows sing with no obvious sexual dimorphism. This goes against the previous examples as the difference, or lack of difference, correlated with the difference in singing behavior between males and females. However, there are multiple examples of no significant correlation between song behavior and the sizes of key song nuclei [10,11,12].
Large-Billed Crow Call:
References:
- Barker, F. K., Cibois, A., Schikler, P., Feinstein, J., Cracraft, J., & Wake, D. B. (2004). Phylogeny and Diversification of the Largest Avian Radiation. Proceedings of the National Academy of Sciences – PNAS, 101(30), 11040-11045. https://doi.org/10.1073/pnas.0401892101
- Brenowitz, E. A., Margoliash, D., & Nordeen, K. W. (1997). An introduction to birdsong and the avian song system. Journal of neurobiology, 33(5), 495-500. https://doi.org/10.1002/(SICI)1097-4695(19971105)33:5<495::AID-NEU1>3.0.CO;2-#
- Nottebohm, F., & Arnold, A. P. (1976). Sexual Dimorphism in Vocal Control Areas of the Songbird Brain. Science (American Association for the Advancement of Science), 194(4261), 211-213. https://doi.org/10.1126/science.959852
- Ball, G. F. (2016). Species variation in the degree of sex differences in brain and behaviour related to birdsong: adaptations and constraints. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1688), 20150117-20150117. https://doi.org/10.1098/rstb.2015.0117
- Gurney, M. E., & Konishi, M. (1980). Hormone-Induced Sexual Differentiation of Brain and Behavior in Zebra Finches. Science (American Association for the Advancement of Science), 208(4450), 1380-1383. https://doi.org/10.1126/science.208.4450.1380
- Brenowitz, E. A., & Arnold, A. P. (1986). Interspecific comparisons of the size of neural song control regions and song complexity in duetting birds: evolutionary implications. The Journal of neuroscience, 6(10), 2875-2879. https://doi.org/10.1523/jneurosci.06-10-02875.1986
- Smith, C. A., Roeszler, K. N., Hudson, Q. J., & Sinclair, A. H. (2007). Avian sex determination: what, when and where? Cytogenetic and genome research, 117(1-4), 165-173. https://doi.org/10.1159/000103177
- Wolf, J. B., & Bryk, J. (2011). General lack of global dosage compensation in ZZ/ZW systems? Broadening the perspective with RNA-seq. BMC genomics, 12(1), 91-91. https://doi.org/10.1186/1471-2164-12-91
- Wang, R., Sun, Y., Zhang, X., Zeng, S., Xie, W., Yu, Y., Zhang, X., & Zuo, M. (2009). Song Control Nuclei in Male and Female Large-Billed Crows (Corvus macrorhynchos). Zoological Science, 26(11), 771-777. https://doi.org/10.2108/zsj.26.771
- Kirn, J. R., Clower, R. P., Kroodsma, D. E., & Devoogd, T. J. (1989). Song‐related brain regions in the red‐winged blackbird are affected by sex and season but not repertoire size. Journal of neurobiology, 20(3), 139-163. https://doi.org/10.1002/neu.480200304
- Brenowitz, E. A., Lent, K., & Rubel, E. W. (2007). Auditory Feedback and Song Production Do Not Regulate Seasonal Growth of Song Control Circuits in Adult White-Crowned Sparrows. The Journal of neuroscience, 27(25), 6810-6814. https://doi.org/10.1523/JNEUROSCI.1248-07.2007
- Ward, B. C., Nordeen, E. J., & Nordeen, K. W. (1998). Individual Variation in Neuron Number Predicts Differences in the Propensity for Avian Vocal Imitation. Proceedings of the National Academy of Sciences – PNAS, 95(3), 1277-1282. https://doi.org/10.1073/pnas.95.3.1277





