Sex differences in the brain refer to variations observed in the structure, function, and organization of the brain between males and females. Research in this field aims to understand whether and how biological factors contribute to cognitive and behavioural differences observed between the sexes. The study of sex differences in the brain has been a topic of scientific investigation for decades, and it continues to be a subject of interest and debate.
History of Sex Differences in the Brain
Historically, the idea of sex difference in the brain was first proposed in the middle of the 19th century by scientists who provided important information regarding the sex difference in the brain. In the 1930s, Pfeiffer conducted an experiment by transplanting ovaries into adult mice and observed recurring activities only in mice that were castrated (deprived of the testes) during the development stage and not in the adult stage (11). In addition, Phoenix et al. in 1959 demonstrated that administering testosterone in prenatal female guinea pigs resulted to usual male behaviours. However, this behaviour was not observed during perinatal or adult stages when testosterone was administered (12).
From these two experiments, this suggests that the main drivers of sex difference in the brain in males and females are determined by the type of gonad (testis or ovaries) produced by the chromosomal constitution (XX or XY), as well as the type of hormone secreted by these organs. Thus, producing the classical model of sex differences in the brain.
In males, the masculinization of the brain results from the conversion of testosterone directly or indirectly to estradiol by an enzyme called “aromatase.” On the contrary, the female brain is protected from masculinization through high levels of alpha-fetoproteins binding to estrogen (Figure. 1) (2; 7; 8).
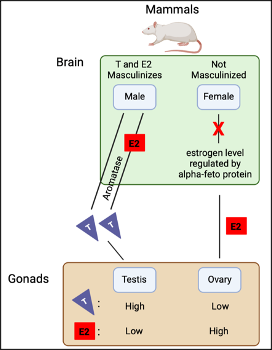
Figure 1. Schematic diagram of sex differentiation in mammals. The steroid hormones produced in the gonads act on the brains to differentiate it into male or female types. In males, there is higher levels of testosterone, whilst in females, testosterone is lower. This results in the masculinization and feminization of the male and female brain, respectively. Abbreviation: Testosterone (T), Estradiol (E2).
However, scientists like Arnold and Breedlove (1), indicated that the organization and activation effect of steroid hormones (classical model) needs to be re-evaluted as different factors are not taken into account. The classical model does not consider difference in gene expression, epigenetics, and expression of sex-biased genes in the male and female brain during different developmental stages. Therefore, hormones may not be the only determining factor when it comes to sex differences in the brain of both males and females.
Difference in Gene Expression During Brain Development
Gene expression refers to a process by which genetic information stored in genes, is used to produce a functional protein. Studies performed on mice and chicken indicated that difference in gene expression was observed during the developing brain before the action of gonadal hormones in the brain (4; 6). This indicates that during brain development, difference in gene expression occurs which leads to the differential development of the male and female brain.
In the scientific literature, the default pathway is suggested to be ovarian differentiation (female development) in the absence of an additional stimulus. In the case of the gonad, which begins as undefined, the additional stimulus is the expression of the SRY (sex-determining region Y). The SRY gene is a master regulator gene located on the Y chromosome and encodes a protein called “testis-determining factor” (TDF) (3). The TDF is known to act as a transcription factor that suppresses the expression of aromatase and promotes the expression of SOX9, leading the to activation of signalling pathways that initiate the development of the testis (Figure. 2). With the development of the testis, this leads to the production of testosterone and other androgen hormones which plays a significant role in masculinizing the brain (Figure. 2) (9; 14).
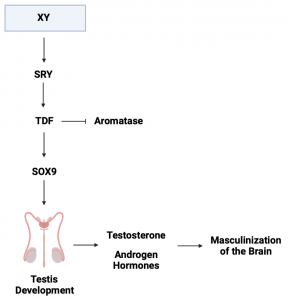
Figure 2. Schematic diagram of testis development, leading to masculinization of the brain. The expression of SRY from the Y chromosome encodes TDF which inhibits the expression of aromatise whilst promoting expression of SOX9. Expression of SOX9 activates cell signalling pathways to initiate testis formation. The testis produces testosterone and other androgen hormones to “masculinize” the brain. Abbreviation: Sex-determining region Y (SRY), Testis-determining factor (TDF).
Epigenetic Sex Differences in the Brain
Epigenetics can be described as modifications that occur on top of the DNA which does not alter the sequence and instead, affect how genes are expressed. Mechanisms of epigenetics include DNA methylation and histone modifications which acts to add methyl groups, as well as the addition or removal of acetyl groups onto the nucleotide base, respectively (5).
In mammalian studies, DNA methylation and histone modifications have been identified as contributors to the sexual differentiation process of the brain. Under normal conditions, activity of DNA methyltransferases (DNMTs) is higher in the pre-optic area (POA) of the hypothalamus in comparison to males where they show the opposite trend (9). However, a study performed in newborn female rats after treatment of masculinizing dose of estradiol, observed a decrease in activity of DNMTs, as well as male-like synaptic pattern in the POA and exhibited male behaviours as adults. Furthermore, genome analyses and whole-genome bisulfite sequencing identified that sex difference between males and females were restricted to high levels of DNA methylation, where female rats had higher percent methylation and fully methylated CpG sites, in comparison to male rats and estradiol-treated female rats (Figure. 3) (10).
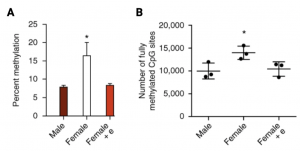
Figure 3. Females have higher DNA methylation in the POA. (A) Females had higher levels of global methylation in the POA in comparison to males and estradiol-treated females. (B) Whole-genome bisulfite sequencing identified that females have more fully methylated sites across the genome compared to males and estradiol treated females. Abbreviation: Pre-optic area (POA).
Overall, from the study above, higher percentage of methylation across the genome and fully methylated CpG sites may correlate to sexual differences in the brain. Moreover, it indicates that numerous amounts of genes are epigenetically repressed during female development and higher levels of steroid in the neonatal male acts to inhibit activity of DNMTs in the POA. This liberates the genes that are required to attain masculinization of both brain and behaviour (Figure. 4).
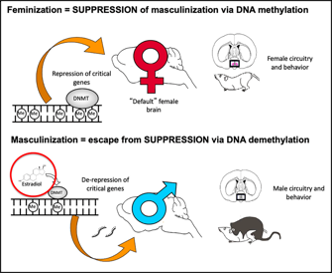
Figure 4. Sex differences in DNA methylation differentiate the POA and adult male sexual behaviour. In females, higher activity of DNMTs lead to the suppression of genes required for masculinization. On the contrary, estradiol leads to decrease activity of DNMTs, resulting in genes required for masculinization escaping methylation. Abbreviation: Pre-optic area (POA), DNA methyltransferases (DNMTs).
Sex-Biased Genes in the Brain During Different Development Stages
Sex-biased genes are genes that exhibit differential expression or function between the males and females. In humans, during different developmental stages, there are sex-biased genes that are differentially expressed in the brain between the two sexes.
A study conducted by Shi et al., 2016, profiling the human brain at major developmental stages, observed expression of sex-biased genes during prenatal, early childhood, puberty, and adulthood. During the prenatal stage, male-biased genes are more expressed in different regions of the brain. Surprisingly, the opposite can be seen during early childhood as each of the brain region is largely driven by female-biased genes. However, during puberty, it reverts back to male-biased genes dominating most of the brain region. In adulthood, no general pattern can be observed as male and female biased genes are equally expressed in different regions of the brain (Figure. 5). Altogether, the brain transciptome displays expression of sex-biased genes in different regions of the brain during development as well as sexual maturation (13).
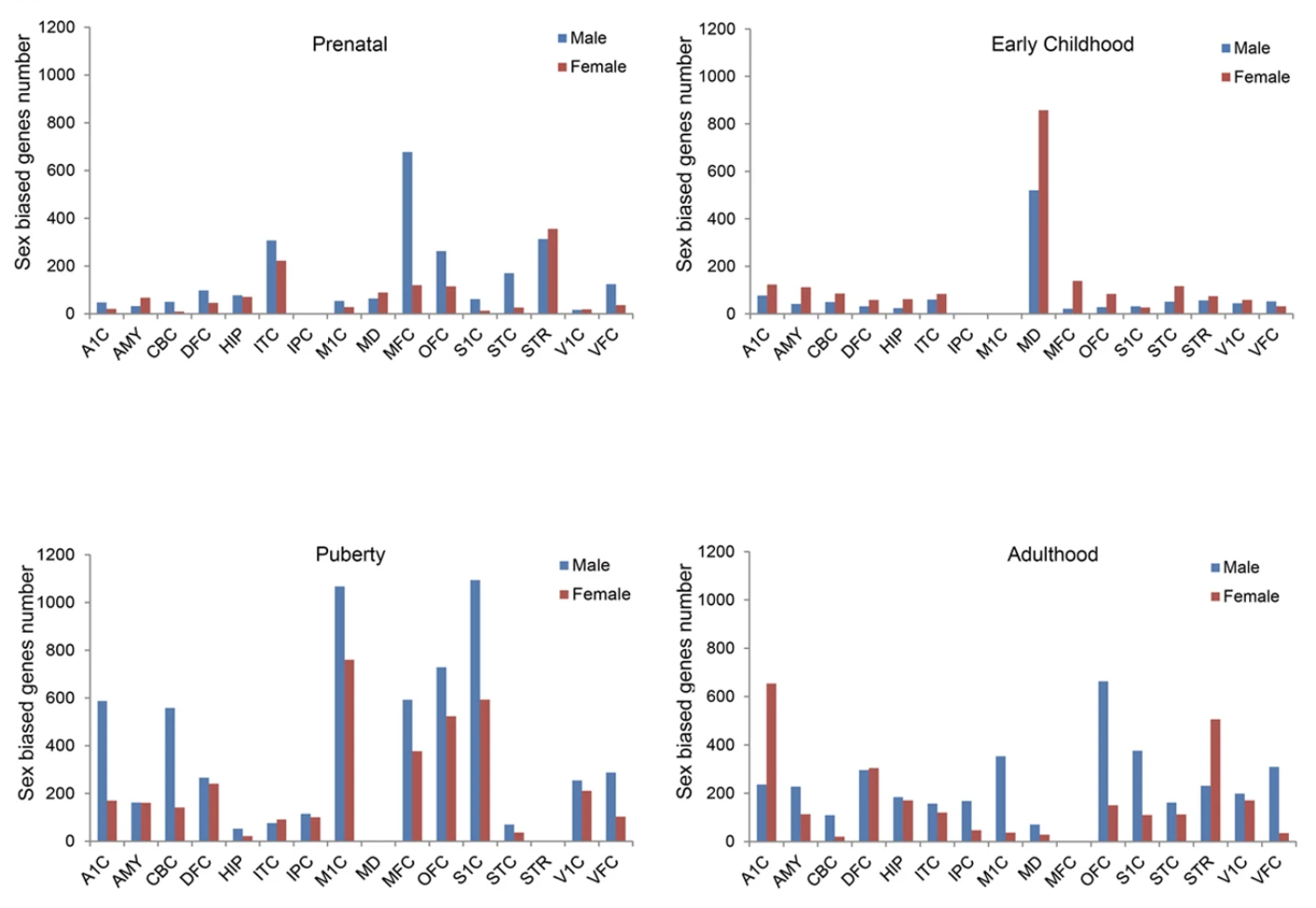
Figure 5. Histogram plot of number of sex-biased genes of each brain region during brain development in the prenatal, early childhood, puberty, and adulthood stage. The studied brain regions include A1C (primary auditory cortex), S1C (primary somatosensory cortex), M1C (primary motor cortex), STR (striatum), DFC (dorsolateral prefrontal cortex), MFC (medial prefrontal cortex), VFC (ventrolateral prefrontal cortex), OFC (orbital frontal cortex), MD (mediodorsal nucleus of thalamus), AMY (amygdaloid complex), ITC (inferolateral temporal cortex), HIP (hippocampus), CBC (cerebellar cortex), V1C (primary visual cortex), STC (posterior(caudal) superior temporal cortex) and IPC (posterior inferior parietal cortex).
Furthermore, enrichment of sex-biased genes involved in brain diseases can also be observed between male and female biased genes. For the male-biased genes, neurological and psychiatric disorders such as autism, bipolar disorder, schizophrenia, Alzheimer’s disease, and Parkinson’s disease, are highly enriched. On the contrary, for the female-biased genes, only a few diseases were hardly significantly enriched such as OCD, schizophrenia, epilepsy, and Alzheimer’s disease (13).
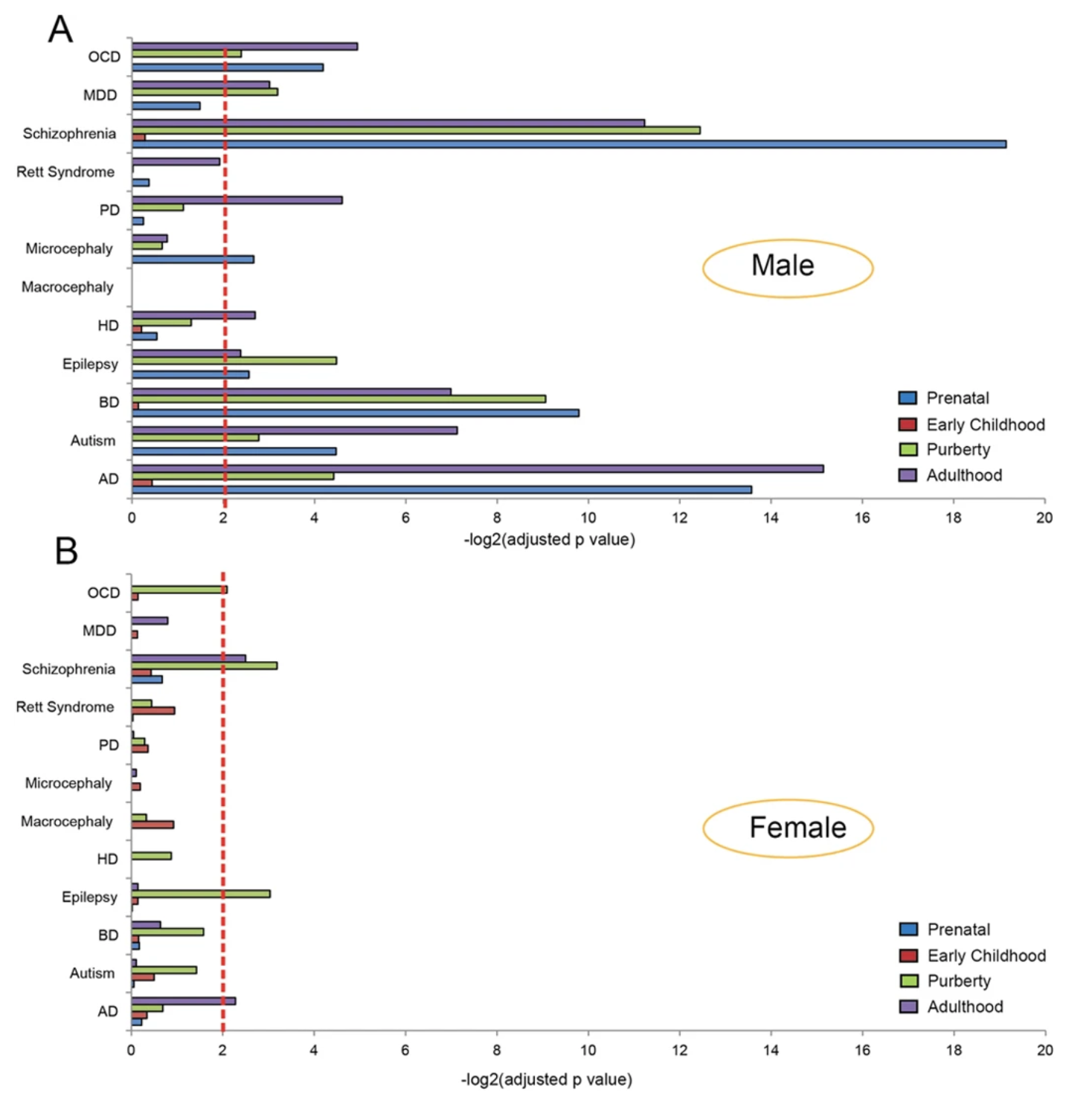
Figure 6. Enrichment scores of the sex-biased genes for brain diseases. Dashed line indicate significance (corrected p-value <0.01). (A) Enrichment of male-biased genes. (B) Enrichment of female-biased genes. Abbreviation: Obsessive compulsive disorder (OCD), major depressive disorder (MDD), Parkinson’s disease (PD), Huntington’s disease (HD), Bipolar disorder (BD), and Alzheimer’s disease (AD).
Altogether, it can be observed that expression of sex-biased genes can be observed between the two sexes during different developmental stages in the brain. During the four developmental stages that were studied, the prenatal and puberty stages are largely dominated by the male biased genes in different regions of the brain. On the contrary, during early childhood, the female biased genes dominate almost all of the different regions of the brain. In addition, expression of sex-biased genes are also involved in both neurological and psychiatric disorders where male-biased genes are highly enriched in comparison to female-biased genes (13).
Perspectives and Other Differences in the Brain
All things considered, the classical model of sex difference in the brain needs to be re-evaluated as it does not take into account the difference in gene expression, epigentics, and expression of sex-biased genes during major developmental stages.
With this in mind, different parts of the brain such the hypothalamus, can also show sex differences in males and females. These differences include structures, the effect of the chromosomal constitution, and epigenetics. Additionally, sex differences in the brain can also be observed in non-mammalian vertebrates such as songbirds, where males sing to court the females.
References
[1] Arnold, A. P., & Breedlove, M. S. (1985). Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Hormones and Behavior, 19(4), 469–498. https://doi.org/10.1016/0018-506x(85)90042-x
[2] Bakker, J., Christelle De Mees, Douhard, Q., Balthazart, J., Philippe Gabant, Josiane Szpirer, & Szpirer, C. (2006). Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. 9(2), 220–226. https://doi.org/10.1038/nn1624
[3] Berta, P., Hawkins, J. B., Sinclair, A. H., Taylor, A., Griffiths, B. L., Goodfellow, P. N., & Fellous, M. (1990). Genetic evidence equating SRY and the testis-determining factor. Nature, 348(6300), 448–450. https://doi.org/10.1038/348448a0
[4] Dewing, P., Shi, T., Horvath, S., & Vilain, E. (2003). Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Molecular Brain Research, 118(1-2), 82–90. https://doi.org/10.1016/s0169-328x(03)00339-5
[5] Dupont, C., Armant, D., & Brenner, C. (2009). Epigenetics: Definition, Mechanisms and Clinical Perspective. Seminars in Reproductive Medicine, 27(05), 351–357. https://doi.org/10.1055/s-0029-1237423
[6] Lee, S. I., Lee, W. K., Shin, J. H., Han, B. K., Moon, S., Cho, S., … Han, J. Y. (2009). Sexually dimorphic gene expression in the chick brain before gonadal differentiation. Poultry Science, 88(5), 1003–1015. https://doi.org/10.3382/ps.2008-00197
[7] MacLusky, N. J., & Naftolin, F. (1981). Sexual differentiation of the central nervous system. Science, 211(4488), 1294–1302. https://doi.org/10.1126/science.6163211
[8] McCarthy, M. M. (2008). Estradiol and the Developing Brain. Physiological Reviews, 88(1), 91–134. https://doi.org/10.1152/physrev.00010.2007
[9] McCarthy, M. M. (2017). Chapter 11 – Sex Differences in the Brain: Focus on Developmental Mechanisms (M. J. Legato, Ed.). Retrieved May 19, 2023, from ScienceDirect website: https://www.sciencedirect.com/science/article/pii/B9780128035061000334
[10] Nugent, B. M., Wright, C. L., Shetty, A. C., Hodes, G. E., Lenz, K. M., Mahurkar, A., … McCarthy, M. M. (2015). Brain feminization requires active repression of masculinization via DNA methylation. Nature Neuroscience, 18(5), 690–697. https://doi.org/10.1038/nn.3988
[11] Pfeiffer, C. A. (1936). Sexual differences of the hypophyses and their determination by the gonads. 58(1), 195–225. https://doi.org/10.1002/aja.1000580112
[12] Phoenix, C. H., Goy, R. W., Gerall, A. A., & Young, W. C. (1959). Organizing Action of Prenatally Administered Testosterone propionate on the Tissues Mediating Mating Behaviour in the Female Guinea Pigs. Endocrinology, 65(3), 369–382. https://doi.org/10.1210/endo-65-3-369
[13] Shi, L., Zhang, Z., & Su, B. (2016). Sex Biased Gene Expression Profiling of Human Brains at Major Developmental Stages. Scientific Reports, 6(1). https://doi.org/10.1038/srep21181
[14] Zhai, G., Jia, J., Bereketoglu, C., Yin, Z., & Pradhan, A. (2022). Sex-specific differences in zebrafish brains. Biology of Sex Differences, 13(1). https://doi.org/10.1186/s13293-022-00442-2


