 Everywhere you look there’s something to stress you: Trump-induced stressing over the end of the world, working insane hours to gain a toe-hold on the housing ladder or to keep food on the table, kids screaming, keeping up with social media, etc…
Everywhere you look there’s something to stress you: Trump-induced stressing over the end of the world, working insane hours to gain a toe-hold on the housing ladder or to keep food on the table, kids screaming, keeping up with social media, etc…
It’s not just your body as a whole that has to deal with stress. Your cells do too. But what your cells consider to be stressful is probably not at the top of your list of things you worry about.
Cells get stressed by things like changes in pH, mechanical stress, heat, UV light and nasty chemicals such as ‘reactive oxidation species’ (ROS).
ROS really like to interact with other molecules. If they are allowed to roam free around a cell, they wreak havoc, damaging proteins and mutating DNA.
We produce them just by staying alive! When cells make energy from food and oxygen, they make ROS as a by-product.
Fortunately, cells have figured out a multitude of different ways of detecting and dealing with things that stress them, and no, they have nothing to do with yoga or mindfulness.
 First of all, a cell has to notice that the stressful thing is there. Usually it does this using a specially shaped detection protein.
First of all, a cell has to notice that the stressful thing is there. Usually it does this using a specially shaped detection protein.
Every cell is full of thousands of different proteins, tiny micro machines, each with its own little job that contributes to keeping the cell staying alive and working properly.
Stress detection proteins have the task of detecting things that are bad for the cell. If a dangerous chemical is around, the detection protein will recognise the chemical by binding to it. The protein will then trigger a molecular alarm signal in the cell, activating other proteins to come and deal with the problem.
In some cases, a cell will even activate an autodestruct sequence, sacrificing itself for the sake of other cells. The name for this cellular suicide is apoptosis.
Dr Peter Mace and his research team have been figuring out how one of these stress detection proteins works.
It’s called Apoptosis Signal-Regulating kinase 1, or ASK1.
ASK1 serves as a molecular switch, converting ROS-induced stress into a signal for the cell to act.
Dr Mace and his colleagues studied the shape and activity of parts of the ASK1 protein. They then made a model of how the protein detects ROS, and passes on the alarm signal to other proteins. And yes, this signal can lead to apoptosis, the cell sacrificing itself.
It helps to think of ASK1 as a tiny machine, with three main connected parts or ‘domains’:
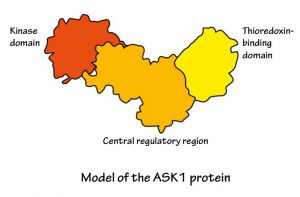 Other researchers have already figured out what the kinase domain part of ASK1 looks like. Dr Mace and his colleagues have found out what the central regulatory domain looks like and how it works.
Other researchers have already figured out what the kinase domain part of ASK1 looks like. Dr Mace and his colleagues have found out what the central regulatory domain looks like and how it works.
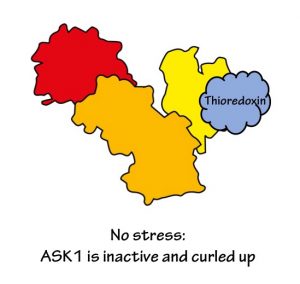
When the cell is happy and not stressed, ASK1 curls up, bringing the two end domains close together. It also sticks to another protein called thioredoxin (using its thioredoxin-binding domain, of course). This all stops ASK1 from being active.
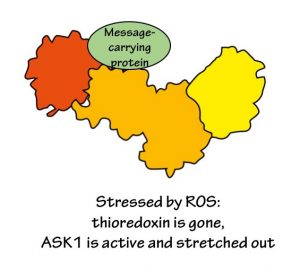
 But when ROS are around, they react with the thioredoxin protein. The change in thioredoxin triggers it to detach from ASK1. This in turn allows ASK1 to stretch out. Once ASK1 is all opened up, other proteins can access the active parts of ASK1. ASK1 modifies these other proteins, and sends them out into the cell, carrying a message for something to be done about the ROS.
But when ROS are around, they react with the thioredoxin protein. The change in thioredoxin triggers it to detach from ASK1. This in turn allows ASK1 to stretch out. Once ASK1 is all opened up, other proteins can access the active parts of ASK1. ASK1 modifies these other proteins, and sends them out into the cell, carrying a message for something to be done about the ROS.
 The whole process is very finely tuned; if ASK1 is activated too much or not enough, bad things happen: for example, mutations that stop ASK1 from working properly can contribute towards Parkinson’s disease, stomach cancer and melanoma. Understanding how ASK1 works will help towards making new drugs that treat these diseases.
The whole process is very finely tuned; if ASK1 is activated too much or not enough, bad things happen: for example, mutations that stop ASK1 from working properly can contribute towards Parkinson’s disease, stomach cancer and melanoma. Understanding how ASK1 works will help towards making new drugs that treat these diseases.
Read the paper here:
Structural basis of autoregulatory scaffolding by apoptosis signal-regulating kinase 1.
Johannes F. Weijman, Abhishek Kumar, Sam A. Jamieson, Chontelle M. King, Tom T. Caradoc-Davies, Elizabeth C. Ledgerwood, James M. Murphy, and Peter D. Mace. Proceedings of the National Academy of Sciences of the U. S. A. 2017; 114(11):E2096-E2105. doi: 10.1073/pnas.1620813114.

