Red Cabbage Indicator
There are a variety of plants that can be used as pH indicators but one of the easiest to find with the widest range of colours is red cabbage. One school we work with have planted a garden with red/purple plants and flowers for the kids to test.
We use it to introduce the concept of acids and bases and where they fit on a scale. It’s also a good introduction into why scientists use numbers eg pH numbers rather than colours (ask a class to point something that is red in the classroom and you’ll see what I mean).
We usually use a set of buffers to create the cabbage pH range:
Citrate buffer
3.0 pH:
Sodium citrate (100 mg , 0.35 mmol) + citric acid (977 mg, 4.65 mmol)
4.0 pH:
Sodium citrate (500 mg, 1.7 mmol) + citric acid (693 mg, 3.3 mmol)
5.0 pH:
Sodium citrate (867 mg, 2.95 mmol) + citric acid (431 mg, 2.05 mmol)
6.0 pH:
Sodium citrate (1.22 g, 4.15 mmol) + citric acid (200 mg, 0.95 mmol)
Sodium phosphate buffer
7.0 pH:
Disodium phosphate (1.73 g, 12.2 mmol) + sodium dihydrogen phosphate (1.08 g, 7.8 mmol)
8.0 pH:
Disodium phosphate (2.69 g, 18.9 mmol) + sodium dihydrogen phosphate (146.2 mg, 1.06 mmol)
Bicarbonate-carbonate buffer
9.0 pH:
Sodium carbonate (212 mg, 2.0 mmol) + sodium bicarbonate (672 mg, 8.0 mmol)
10 pH:
Sodium carbonate (742 mg, 7.0 mmol) + sodium bicarbonate (252 mg, 3.0 mmol) –
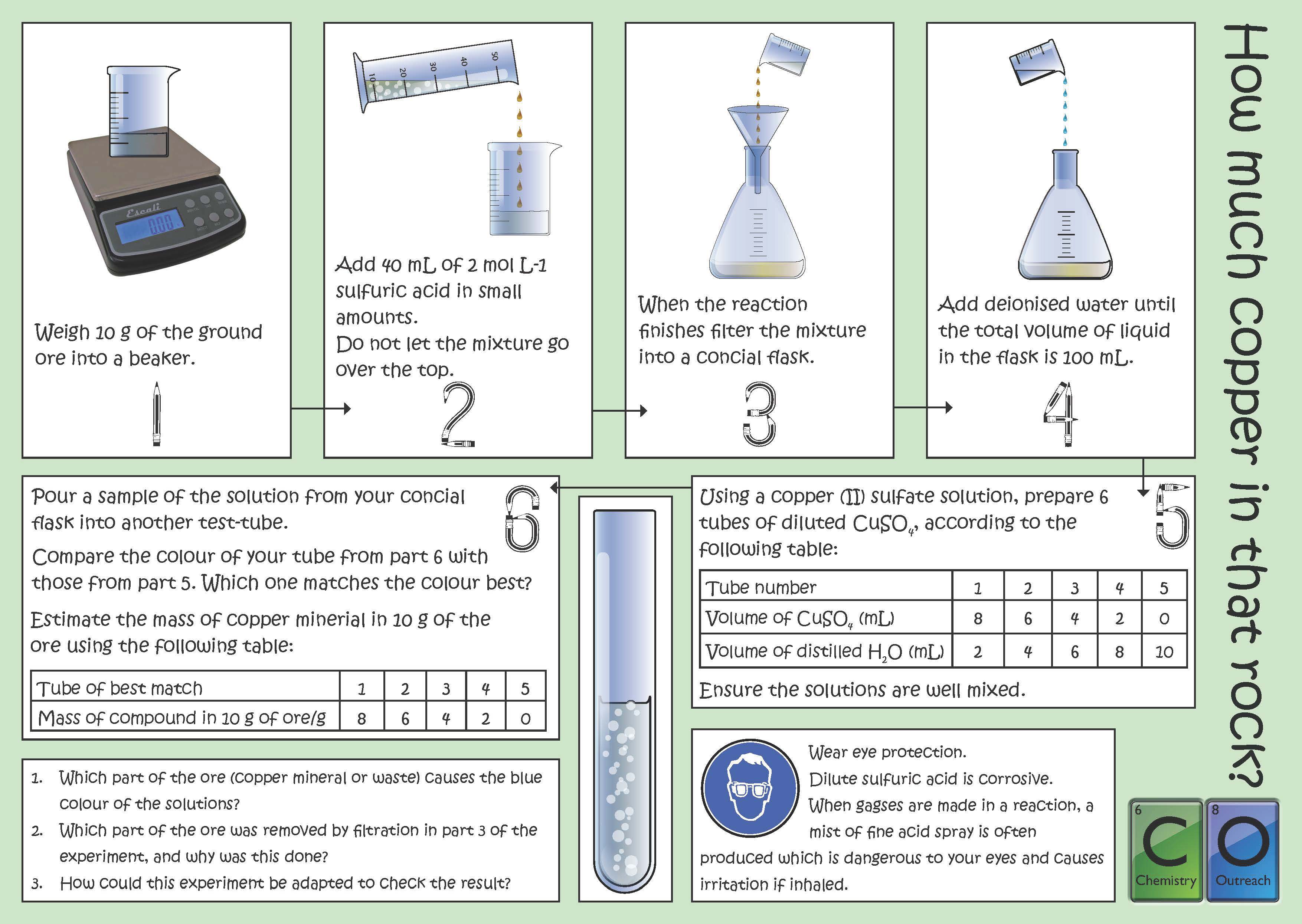
Copper Ore
Colorimetry is a simple technique based that can be used to teach basic measuring skills. When used in a context such as “the amount of copper in a copper ore” the back story adds to the learning experience and can be expanded to include things such as environmental issues. The colours and use of simple apparatus engages the school pupils and they really engage well with the activity.
This experiment is based on one found in the Royal Society of Chemistry book ‘Classic chemistry experiments’. We have modified it a little and often play a game at the end looking at the likely impact of a mine on an area and the considerations that need to be factored in when decisions like this are made.
In the basic experiment students measure the colour of the ‘ore’ sample solution by eye but in recent times we have started using smart phone apps to bring technology into the classroom.
Although there are safety issues with using the sulfuric acid, we have used this with year 5 and 6 pupils. However, these classes are part of our program and have had a few years of working with us and handling glassware and chemicals before we progress to this level of work.
Story telling with chemistry
A few years ago we had some fun with Steve Ting, a teaching fellow over in Science Communication. We spent a few days making videos, looking at how story telling could be used to explain research. The results were really fun and we had a great time making them:
The Granny Smith Affair
The use of Isotopes to identify the country of origin of fruit
Raman
A Darker video about the role that Raman spectroscopy can play in the field of criminal forensics
What’s broccoli good for?
A film made for a competition about nutrition
Five signs that you are a chemist
A tongue in cheek look at being a chemist
Measuring the density of liquids
One of our basic learning activities is to measure the density of liquids. We use small balances that we can buy off the internet for a few dollars and 10 mL measuring cylinders. Typically the activity starts with a discussion of floating and sinking (sometimes using a helium balloon and liquid nitrogen to change it’s density) and then arrives at the question what is density. In simple terms we talk about ‘how much something weighs for it’s size’ (we use mass and volume but sometimes have to over simplify-apologies to the physicists).
To allow comparison between liquids we control the volume, so the kids measure 10 mL of each liquid. Water (green food dye), canola oil, methanol (red food dye) and saturated copper sulfate solution.
This video shows it a class at Tahuna Intermediate, Dunedin (for this class we also included hexane as a, short lived, experiment).
Quite often we allow the lesson to run after the stage in the video and set a challenge to create as many layers as possible (mixing methanol and water/copper sulfate creates a layer with new density).
The most we have ever seen in 10 years of doing this experiment is 15 layers, keeping layers of water and methanol (and copper sulfate) separated with layers of oil. The narrow 10 mL measuring cylinders are important to keep the layers separate.




Recent comments